Background:CEBPA-mutated acute myeloid leukemia (AML) is a separate entity in the updated WHO and ICC classifications. Previously identified by double mutations, the clinical profile and favorable outcome were correlated with in-frame mutations in bZIP domain. Beyond mutations recurrent in myeloid neoplasms (involving TET2, FLT3, NRAS), CEBPA-mutated AML is enriched in gene mutations (i.e., GATA2, CSF3R, WT1) rarely observed in CEBPA-wild type AML. No conclusive findings have been drawn for the prognostic impact of additional mutations. Of note, somatic GATA2 mutations have been associated with invasive fungal infections in myeloid neoplasms. Although overall correlating with favorable prognosis, an unexpectedly high rate of treatment-related mortality (TRM) in CR has been described in a large multicenter study on intensively treated CEBPA-mutated patients (pts) (Schlenk et al Blood 2013;122(9):1576).
Aims: The aim of our study was to correlate baseline and treatment characteristics with the outcome and hematological toxicity in an intensively treated cohort of CEBPA-bZIP mutated AML pts.
Methods: Pts entering the study had a diagnosis of CEBPA-bZIP AML confirmed according to 2022 WHO and ICC criteria. Pts were characterized by next-Generation deep amplicon sequencing with Ion Torrent platform (ThermoFisher Scientific) covering a panel of 40 genes. Sequence alignment and filtering were performed by NextGENe version 2.4.2.1 (SoftGenetics). The first two chemotherapy cycles were categorized according to the delivery of an anthracycline (ANTHRAC) and cytarabine (ARA-C) at high dose (HDAC). The study was approved by the local institutional review board.
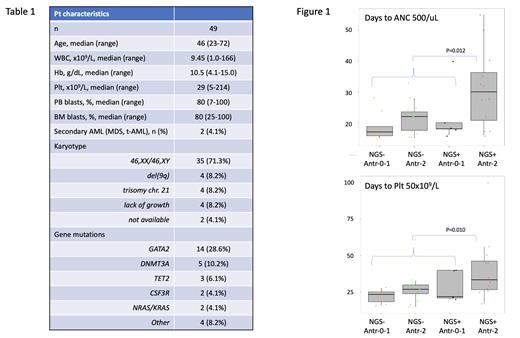
Results: From 2004 to 2023, 49 pts with CEBPA-bZIP mutated AML met the inclusion criteria at project study sites (Firenze, Bergamo). Their features are detailed in Table 1. CR rate was achieved in 45 of 49 pts (91.8%), with a DFS and OS of 74.3% and 82.4% at 5 y, respectively. Based on NGS, at least one additional mutation was identified in 18 (36.7%) pts (NGS+), the majority of them involving GATA2 (n=14, 28.6%). As per treatment, 44 pts received at least two cycles, of which no (n=2, 4.6%), one (n=14, 31.8%), or two (n=28, 63.6%) ANTHRAC-containing cycles. As regarded HDAC, 19 (43.2%), 17 (38.6%) and 8 (18.2%) pts received no, one or two HDAC-containing cycles, respectively. No significant differences emerged for DFS or OS between NGS+ and NGS- groups (P=0.33 and 0.52, respectively). To gain insight into potential causes of toxicity during CR, we focused on hematopoietic recovery after induction and consolidation. NGS+ pts showed slower neutrophil (ANC) recovery (median, 22 days to >500/uL after induction and 25 days after consolidation) compared to NGS- group (19 days, P=0.018; 18 days P=0.058, respectively). Such effect was enhanced by treatment with ANTHRAC-containing cycles: NGS+ pts receiving two cycles required significantly longer time to recover from first consolidation cycle, both for ANC (30 days) and platelet count (34 days) vs 22 (P=0.012) and 27 (P=.010) days, respectively, of other categories (Figure 1). A proportion of 45.5% (5/11) NGS+/ANTHRAC-2 pts could not complete the planned chemotherapy program due to hematological toxicity compared to 19.3% (6/31, P=0.09) in other categories. No significant prolongation of recovery was observed for HDAC.
Conclusions:CEBPA-bZIP mutated AML is featured by high response rate and favorable outcome. Delayed hematopoietic recovery was observed in pts bearing additional mutations (especially GATA2) when treated with anthracyclines in first two cycles. Our findings suggest the cumulative dosage of anthracyclines should be limited in CEBPA-mutated/NGS+ patients to spare hematological toxicity on turn impairing the therapeutic plan.
Disclosures
Frigeni:Jazz Pharmaceutical: Honoraria; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Guglielmelli:GSK: Speakers Bureau; Novartis: Other: Other member of advisory board, speaker at meeting, Speakers Bureau; Abbvie: Other: Other member of advisory board, speaker at meeting, Speakers Bureau. Rambaldi:Abbvie: Honoraria. Vannucchi:GSK: Honoraria; BMS: Honoraria; Abbvie: Honoraria; Roche: Honoraria; AOP: Honoraria; Novartis: Honoraria; Incyte: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal